Take Control of Your Global Patient Recruitment
Centralize recruitment with unprecedented transparency
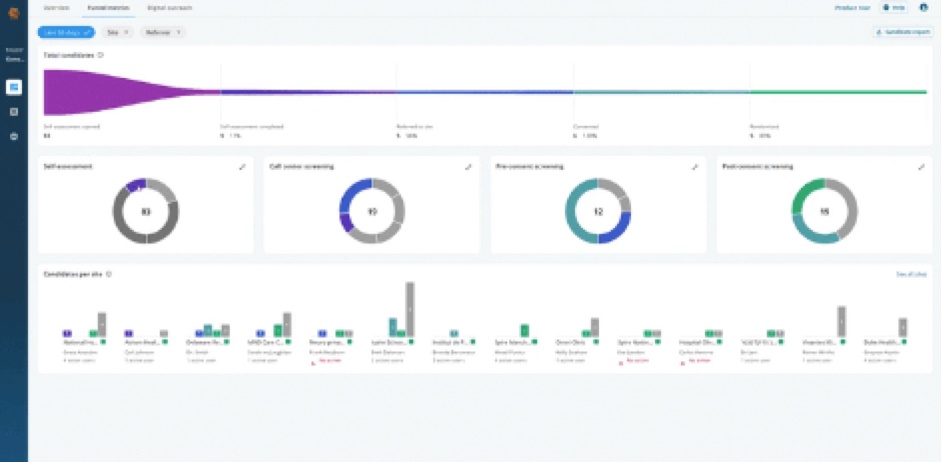
- All referrals in a single funnel
- Data standard across vendors
- Visual ROI metrics for every partner
- Actionable site and candidate insights
Connect and qualify ideal patients for your trial

- Data-driven patient profile modeling
- Live medical secondary screening
- Hyper-targeted digital outreach
- Hands-on global success guidance
Meet patients where they are in their journey

- Curated ecosystem of proven partners
- Diversity and community engagement
- New and unique patient pathways
- Robust ROI reporting in Honey

Recruitment Insights
Patient Recruitment Solutions Trusted By…
Be part of a dynamic team driving innovation and success
Trialbee is growing – and we’re looking for driven, talented, and passionate team members who deeply care about improving clinical research access and enrollment outcomes for all patients worldwide.